RNA Therapies
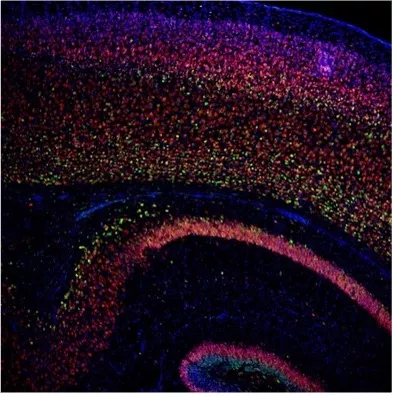
Evaluating the safety, biodistribution, and efficacy of prenatal therapies with antisense oligonucleotide) (ASO).
We are evaluating safety, biodistribution, and efficacy of prenatal ASO (antisense oligonucleotide) therapies for the treatment of neurologic conditions such as Angelman Syndrome (AS) and Spinal Muscular Atrophy (SMA). Antisense oligonucleotides (ASOs) are disease-modifying agents made of short DNA:RNA strands that can treat numerous conditions by regulating alternative splicing, degradation or translation of mRNA or pre-mRNA. We are examining the efficacy and feasibility of a prenatal ASO therapy for severe neurological conditions including Angelman Syndrome (AS) and Spinal Muscular Atrophy (SMA). In our lab we have established a mouse model of in utero delivery to understand the efficacy of various ASOs to treat AS and SMA. We also perform testing in a fetal sheep model to understand the safety and biodistribution of prenatal ASO administration. In addition to the therapeutic outcomes, we are also using these models to better understand the blood brain barrier during fetal development and to optimize therapeutic delivery to the central nervous system. Our ultimate goal is to safely bring these prenatal ASO therapies to a phase I clinical trial.
